Abstract
Purpose: The prevention of relapse is the major therapeutic challenge in older patients with acute myeloid leukemia (AML) who have obtained a complete remission on intensive chemotherapy. There is no established post-remission treatment for prevention of relapse in this setting, except allo HSCT. This randomized phase III study (HOVON97) in older patients (≥ 60 years) with AML or MDS-RAEB in CR/CRi after at least 2 cycles of intensive chemotherapy assessed the value of azacitidine as post remission therapy (in comparison to observation) with respect to the disease free survival (primary endpoint) and overall survival (secondary endpoint).
Patients and methods: In total 117 patients were randomly 1:1 assigned to either observation (control group ) or azacitidine maintenance (aza group), 50 mg/m2, s.c., day 1-5, q 4 weeks, until relapse for a maximum of 12 cycles. The study was powered to detect improvement in disease free survival (DFS) after 1 year from 40% to 60% (power 80%, two-sided test at 5% significance). The current pre-final evaluation after study closure is based on the first 109 patients with at least one year follow-up. The 2 arms (56 control patients vs 53 aza maintenance patients) were comparable with regard to age (60-81 years, median 69 years), performance status, peripheral blood counts and cytogenetics. Although all patients had < 5% bone marrow blasts at randomization, one patient in the aza group had a relapse prior to start aza maintenance.
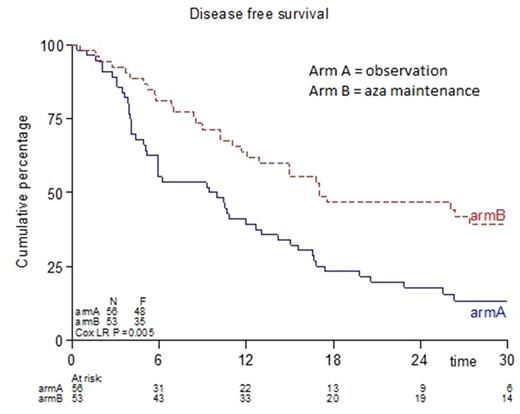
Results: A total of 52 patients received at least 1 cycle of aza. Subsequently, 44, 40, 34 and 32 patients received at least 3, 6, 9 and 12 cycles respectively. The maintenance treatment with aza was feasible, as, during the first 4 cycles, most treatments could be given according to schedule (87% < 30 days interval and 9% 30-40 day interval), at full dose (98%) and without transfusions (95%). The absolute number of AE's grade ≥ 2 were 417 in the control group and 474 in the aza group. The difference in DFS between the two arms was statistically significant in the cohort of patients in this pre-final analysis (Cox regression; p=0.005). The 12 months DFS was estimated at 39% for the control group and at 63% for the aza group. The difference in overall survival (OS) between the two groups currently is not statistically significant in the cohort of patients in this pre-final analysis (Cox regression; p=0.35). In total 31 patients in the control group and 11 patients in the aza group received therapy while off protocol after relapse (control group vs aza group: 7 vs 2 patients received azacitidine; 18 vs 5 patients received other chemotherapy; 6 vs 4 patients received other treatment; 8 vs 1 patients received allogeneic hematopoietic cell transplantation (HCT)). One of the control patients received allogeneic HCT in CR. After censoring patients who received an allogeneic HCT (n=9 and n=1 in control group and aza group respectively) the difference in OS was statistically different (Cox regression; p=0.04). The 12 months OS (after censoring allo transplanted patients) was estimated at 64% for the control group and 83% for the aza group. A planned subgroup analysis (CR vs CRi at inclusion) revealed that patients with platelet count ≥ 100 x 109/L had a significant better OS in favor of aza maintenance treatment (logrank; p=0.01).
Conclusion: Post-remission treatment with aza in older AML patients in CR/CRi after at least 2 cycles of intensive chemotherapy significantly improves DFS (p=0.005). When patients who received an allo HSCT were censored at time of transplant, the difference in OS between both arms was also significantly different (p=0.04), in favor of aza maintenance treatment
Huls: Celgene: Consultancy; J&J: Consultancy. Kuball: Miltenyi: Research Funding; Gadeta (www.gadeta.nl): Consultancy, Equity Ownership, Patents & Royalties: on gdT cells and receptors and isolation strategies , Research Funding. Ossenkoppele: J&J: Consultancy, Honoraria; Celgene: Honoraria, Research Funding; Roche: Honoraria; Karyopharm: Consultancy, Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.